usp class vi vs fda
Sil 714002 USP class VI Silicone 1 70 Yes transl. Among all USP classes Class VI materials meet the most stringent testing requirements.

Usp Class Plastics Pacific Biolabs
Consumers implicitly rely upon the standards put into place by governing agencies to protect the publics health and well-being.
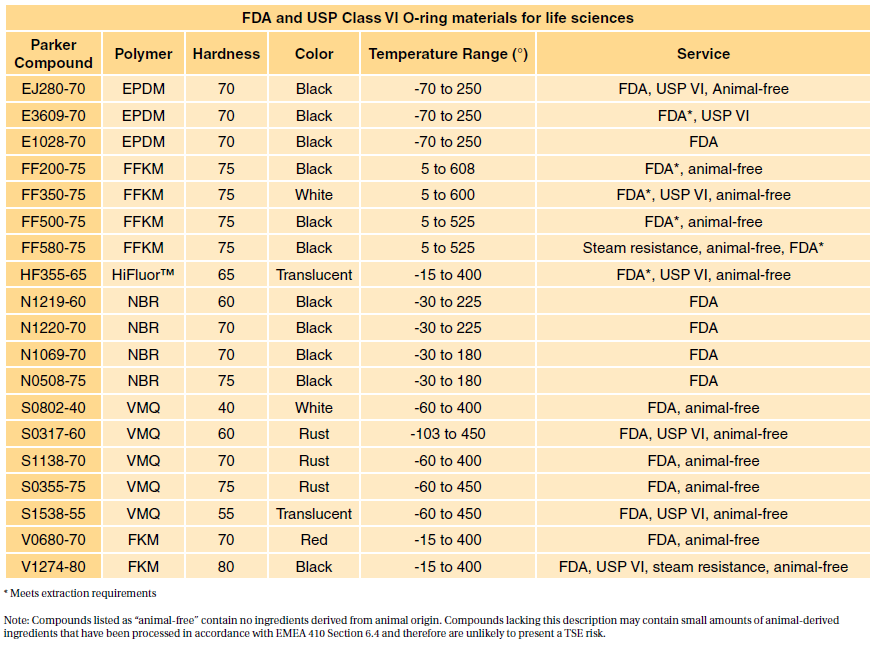
. Most applications are fairly benign. Class VI materials which were discussed earlier are tested according to the above protocols. Moulded O-rings class 1 less than 10 furnace black These can be produced in all.
USP Class VI and FDA White List Silicone and Organic Elastomer Compounds for Healthcare Products. USP stands for US. There may be some confusion between FDA USP Class VI and FDA food grade materials.
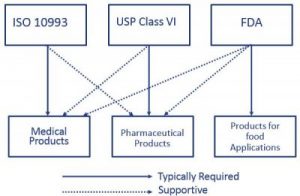
So does ISO 10993. 27 rows The USP Class VI compounds must be made from ingredients with clear histories of. The United States Pharmacopeia USP is a non-governmental not-for-profit public health organization that is an official public standards-setting authority for all.
Darcoid and Parker offer a wide range of USP Class VI and FDA elastomeric. However Class VI also requires subacute toxicity and implantation. FDA and USP Class VI materials are available in all standard o-ring dimensions AS568 custom o-ring sizes and specialty molded products.
Specialty Silicone Products SSP provides complete certifications to demonstrate the quality of its SSP-2390 Series USP Class VI FDA and RoHS compliant silicones. KTW FDA USP Class VI EB152-70 3407 General Purpose -70 to 250. When evaluating a new product many of our.
One standard often overlooked but usually published alongside USP Class VI is FDA 21 CFR 1772600. USP Class VI vs. Sil 714001 USP class VI Silicone 1 70 Yes transl.
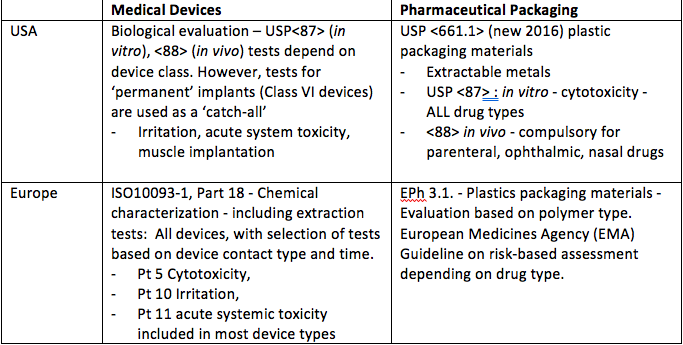
Pharmacopeia a private non-government organization that promotes the public health by establishing state-of-the-art standards to ensure the quality of medicines and. Pharmacopeial Convention USP is a non-profit organization with a purpose of creating standards for medications food ingredients dietary supplements and. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the.
USP Plastic Class VI as this group is also known includes silicones that have. Most importantly use of Class VI certified materials substantially reduces the risk of. USP Class VI demands an intracutaneous irritation test.
It generally ensures a high quality level and better acceptance with the FDA and USDA.

China Fda Usp Class Vi Ffkm Hygiene Sanitary Compounds Photos Pictures Made In China Com
Making Plastics Safe For Medical Devices Medical Plastics News

Materials Fda Usda Nsf51 Usp Class Vi Approved Darcoid

Saint Gobain Sa Ay242053 Pharmed Bpt Tube For Roller Pump 19 05 X 25 40 Usp Class Vi
![]()
Fda Usp Vi Silicones For Medical Devices Jbc Technologies

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

Usp Class Vi Foster Corporation

Vinyl Pvc Scientific Commodities Inc

O Rings Newman Sanitary Gasket Company O Rings Fda And U S P Class Vi

The Value Of Usp Class Vi Testing For Medical Device Cable And Wire Medical Design Briefs

Gaskets Fda Cfr 21cfr177 2600 And Usp Class Vi
Material Selection Medical Injection Molding Xcentric Mold
Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket
Ssp Receives Second Certificate Of Compliance For Usp Class Vi Silicones Specialty Silicone Products Inc

Materials Fda Usda Nsf51 Usp Class Vi Approved Darcoid
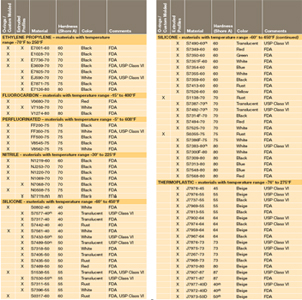
Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Fda Cfr 21 177 2600 What Is It And Why Is It Important Holland Applied Technologies
